What Is Litfulo And How Does It Treat Hair Loss Effectively?
Litfulo is an oral prescription medication developed to treat autoimmune-related hair loss, particularly alopecia areata.
By targeting the immune pathways responsible for follicle damage, Litfulo helps reduce inflammation and allows hair follicles to resume natural growth over time.
What Is Litfulo?
Litfulo is the brand name for ritlecitinib, a selective inhibitor of Janus kinase 3 (JAK3) and TEC family kinases. It is designed to regulate abnormal immune responses rather than stimulate hair growth directly.
The treatment is approved for individuals aged 12 and older with severe alopecia areata, a condition in which the immune system mistakenly attacks hair follicles.

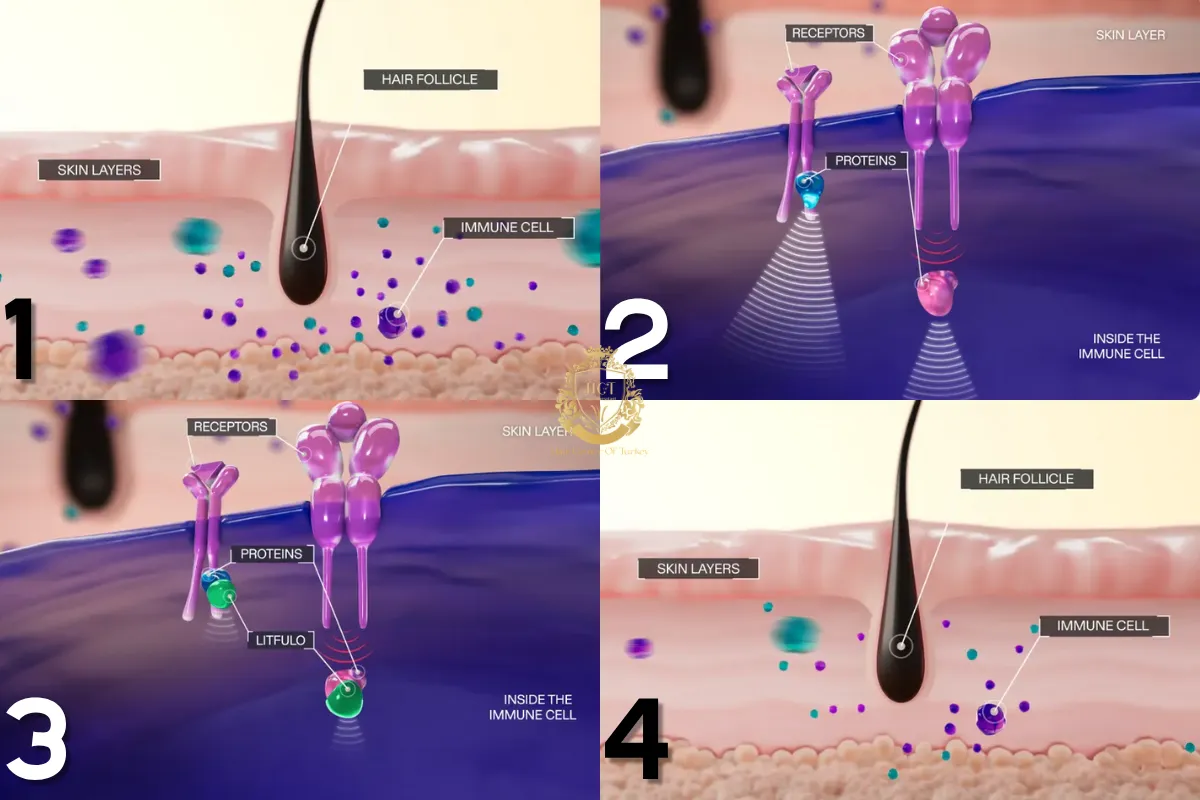
How Litfulo Works In The Body
Alopecia areata is driven by immune signaling that disrupts the hair growth cycle. Litfulo works by interrupting these signals, reducing inflammation around the hair follicle.
By calming the immune response, follicles that were forced into a resting state may gradually return to the growth phase. This approach addresses the underlying cause rather than masking symptoms.
Who Is Litfulo Intended For?
Litfulo is prescribed for patients with moderate to severe alopecia areata. This includes patchy hair loss as well as more extensive forms such as alopecia totalis or universalis.
It is not indicated for androgenetic alopecia or age-related pattern hair loss. Its use should always be guided by a qualified healthcare professional with experience in autoimmune conditions.
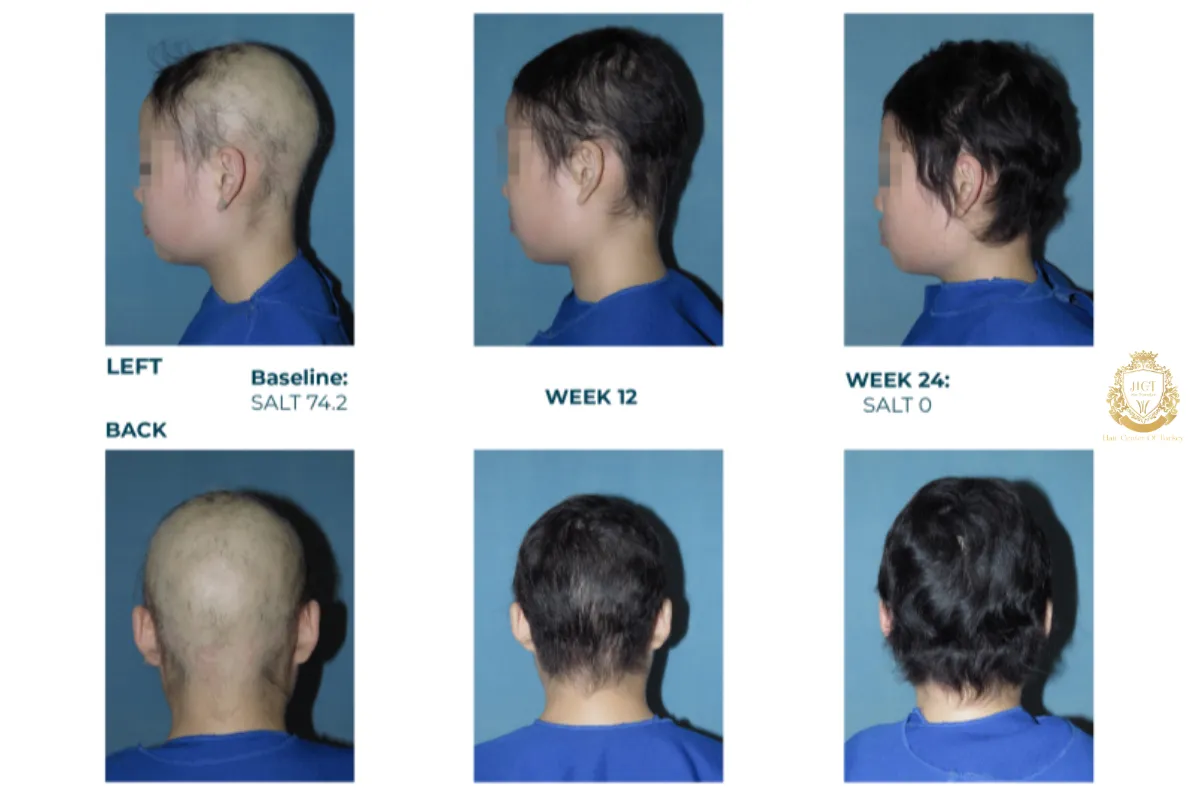
Clinical Results And Effectiveness
Clinical studies have shown meaningful regrowth in a significant number of patients within 24 weeks of consistent use. Some participants achieved substantial scalp coverage even after long-standing hair loss.
While results vary, these outcomes represent a major development for individuals who previously had limited treatment options.
How Litfulo Is Taken
Litfulo is taken orally once per day. Its tablet form allows for easy integration into daily routines without the need for injections or topical applications.
Because it affects immune function, ongoing medical supervision is required. Regular follow-ups help ensure both safety and effectiveness throughout treatment.
Advantages Compared With Traditional Treatments
Litfulo differs from older therapies in several ways:
It targets immune-driven hair loss at the source
It offers systemic treatment rather than localized symptom relief
It avoids long-term corticosteroid exposure
It is suitable for adolescents as well as adults
These factors make it a meaningful option for appropriately selected patients.
Limitations And Safety Considerations
Litfulo is not suitable for all forms of hair loss. It may also cause mild side effects such as headaches, acne, or upper respiratory infections.
Because the medication is relatively new, long-term data is still being collected. Cost and insurance coverage may also influence accessibility in some regions.
The Role Of Litfulo In Future Hair Loss Treatments
Litfulo reflects a shift toward targeted, immune-based therapies in dermatology. Its success has opened the door for further research into precision treatments for autoimmune hair disorders.
As clinical experience grows, treatment protocols may become more refined, potentially expanding its role within hair loss management.
Frequently Asked Questions
Is Litfulo officially approved for hair loss treatment?
Yes. Litfulo is approved for treating alopecia areata in patients aged 12 and older under medical supervision.
How long does it take to see results?
Many patients begin noticing regrowth between 12 and 24 weeks, though individual responses differ.
Can Litfulo treat genetic hair loss?
No. Litfulo is not designed for androgenetic or hormone-related hair loss conditions.
Is Litfulo safe for adolescents?
Yes. It is one of the few treatments approved for individuals as young as 12 with severe alopecia areata.
Can Litfulo be combined with other treatments?
In some cases, combination approaches may be considered, but this should only be done under specialist guidance.




